Clinical Research Coordinator Resume Examples for 2025
Create Your Resume NowIn the world of clinical trials and medical research, a clinical research coordinator is the linchpin that holds everything together. Crafting a standout clinical research coordinator resume is your ticket to landing a role where precision, organization, and scientific acumen are prized. In this article, you'll find expert tips, clinical research coordinator resume examples, and templates to guide you on your path to success. Let’s begin!
This guide will show you:
- A clinical research coordinator resume example that’s better than 9 out of 10 other resumes.
- How to write a clinical research coordinator resume that will land you more interviews.
- Tips and examples of how to put skills and achievements on a clinical research coordinator resume.
- How to describe your experience on a resume for a clinical research coordinator to get any job you want.
Want to save time and have your resume ready in 5 minutes? Try our resume builder. It’s fast and easy to use. Plus, you’ll get ready-made content to add with one click. See 20+ resume templates and create your resume here.
Sample resume made with our builder—See more resume examples here.
One of our users, Brittanya, had this to say:
Zety really helped me create the best resume possible. It pointed out how things could be better on my existing resume and suggested many things to be re-worded or removed.
Want to check more samples related to the clinical research coordinator profession? Here you go:
- Scholarship Resume Examples
- Healthcare Resume Examples
- Public Health Resume Examples
- Administrative Coordinator Resume Examples
- Teaching Assistant Resume Examples
- PhD Resume Examples
- Lab Assistant Resume Examples
- Scientific Resume Examples
- Lab Technician Resume Examples
- Best Sample Resumes for All Jobs
Clinical Research Coordinator Resume Sample
Taylor J. Morgan
Clinical Research Coordinator
(555) 876-5432
taylorjmorgan@email.com
linkedin.com/in/taylor-j-morgan
Summary
Organized and analytical Clinical Research Coordinator with 6+ years of experience managing clinical trials and ensuring compliance with regulatory standards. Skilled in patient recruitment, data collection, and protocol adherence. Passionate about advancing research initiatives and eager to support groundbreaking studies at Clinix.
Experience
Clinical Research Coordinator
City Hospital Research Center, New York, NY
April 2021–November 2024
Key Qualifications & Responsibilities
- Coordinated multiple clinical trials, managing participant recruitment, informed consent processes, and ensuring protocol adherence.
- Monitored patient data and recorded adverse events, ensuring accuracy and compliance with Good Clinical Practice (GCP) guidelines.
- Collaborated with principal investigators, sponsors, and regulatory authorities to maintain study integrity and streamline workflows.
- Prepared regulatory documents and assisted in Institutional Review Board (IRB) submissions, maintaining thorough documentation for audits.
Key Achievement:
- Successfully improved participant retention rates by 20% through personalized follow-ups and enhanced communication strategies.
Clinical Research Assistant
Medical Innovations Institute, Boston, MA
June 2018–March 2021
Key Qualifications & Responsibilities
- Supported clinical trials by screening and enrolling study participants, collecting data, and monitoring compliance with study protocols.
- Conducted patient follow-ups and documented outcomes, ensuring data accuracy for analysis.
- Assisted in preparing study documentation, including consent forms and data collection sheets, and maintained organized trial records.
Key Achievement:
- Recognized for accuracy and attention to detail, contributing to a flawless audit outcome during a critical study phase.
Education
Bachelor of Science in Biology
Boston University, Boston, MA
September 2014–May 2018
Skills
- Clinical Trial Coordination
- Patient Recruitment & Consent
- Data Collection & Management
- Protocol Adherence
- Regulatory Compliance
- Good Clinical Practice (GCP)
- IRB Submission & Documentation
- Communication & Collaboration
Certifications
- Certified Clinical Research Coordinator (CCRC), Association of Clinical Research Professionals (ACRP), 2021
- Good Clinical Practice (GCP) Certification, 2021
Languages
- English—Native
- French—Intermediate
Here’s how to write your own clinical research coordinator resume:
1. Format Your Clinical Research Coordinator Resume Template Correctly
In the competitive field of clinical research, it's essential to make your resume stand out. To capture the attention of hiring managers, select a good resume format that showcases your qualifications clearly and professionally.
To format your resume for clinical research coordinator positions:
- Start with a resume header featuring your name, phone number, email, LinkedIn profile, and any relevant online portfolio links. These are the critical personal details in a resume.
- Including your street address is optional, but listing your city can be advantageous if it matches the job location.
- Choose a reverse-chronological resume format which highlights your most recent job first. This resume type is preferred by employers as it follows the best layout for resumes.
- Select a professional resume font such as Calibri or Arial. Keep the font size between 10 to 12 points.
- When saving your resume, name your resume file “Your Name - Clinical Research Coordinator - Resume.pdf.” A PDF resume file ensures your layout remains intact.
- Typically, the ideal resume length is one page, especially for entry-level roles. However, if you have significant experience, a two-page resume may be suitable.
Read more: How to Update Your Resume
2. Customize Your Clinical Research Coordinator Job Description for a Resume
Tailoring your resume to a specific job is vital because generic resume experience sections often fail to capture interest. If a hiring manager only sees a generic list of duties, they might question the depth of your expertise.
Here’s how to effectively add relevant experience to your resume:
- Use the exact job title from the job advertisement to make your resume ATS-friendly.
- After listing the company name and your employment dates, include 3–6 bullet points. (More for recent positions, fewer for older ones.)
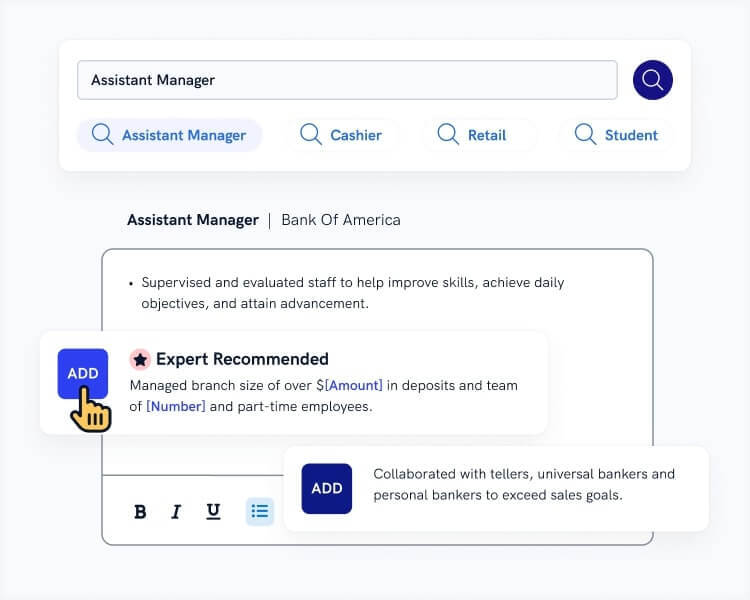
- Highlight how you applied your skills with plenty of achievements. The best achievements to put on a resume are quantifiable.
- Begin statements with strong resume action verbs like coordinated, designed, and prepared.
Clinical Research Coordinator Examples: Responsibilities for a Resume
- Conduct clinical trials in accordance with study protocols and regulatory requirements.
- Recruit, screen, and enroll participants, ensuring informed consent is obtained.
- Coordinate communication between study sponsors, investigators, and research teams.
- Maintain accurate and timely documentation of study activities and data collection.
- Ensure compliance with Good Clinical Practice (GCP) guidelines and ethical standards.
- Monitor participant progress and report adverse events as necessary.
- Assist in the preparation and submission of regulatory documents to the Institutional Review Board (IRB).
Remember to use action verbs that convey your responsibilities and achievements effectively. Here are some action verbs for a clinical research coordinator:
Clinical Research Coordinator Resume Examples: Action Verbs
- Coordinated
- Implemented
- Monitored
- Analyzed
- Documented
- Facilitated
- Managed
- Conducted
- Ensured
- Assisted
Read more: How to List Projects on Your Resume
3. Make Your Education Section Count
While everyone lists their educational background on a resume, simply mentioning your degree, institution, and dates is not enough. To truly stand out, leverage your education to demonstrate additional skills and expertise, increasing your chances of securing interviews.
Consider these resume tips:
- Ideally, place your degree details right after your work experience.
- For entry-level resumes, include relevant coursework to showcase your competencies.
- Even with professional experience, mentioning fellowships, scholarships, or leadership roles can add value.
- Wondering if you should list your GPA on a resume? Include it if it is impressive.
Read more: Presenting Your Major and Minor on a Resume: Tips & Examples
Creating a resume with our builder is incredibly simple. Follow our step-by-step guide and use content from Certified Professional Resume Writers to have a resume ready in minutes.
When you’re done, Zety’s resume builder will score your resume and our resume checker will tell you exactly how to make it better.
4. Prove the Clinical Research Coordinator Skills the Company Wants
Your resume needs to highlight skills the employer seeks, but there's a strategic way to do it. While you might know some essential clinical research coordinator skills, choosing them randomly might not make your resume stand out. Instead, focus on the key soft and hard skills listed in the job posting.
Here's how to list clinical research coordinator skills on a resume:
- The skills mentioned in the job ad serve as important resume keywords. Incorporate these into your resume's skills section.
- Avoid overwhelming your resume with too many skills. A concise list helps the essential ones shine.
- To increase your chances of getting an interview, ensure these skills are reflected in your work and education descriptions.
Clinical Research Coordinator Resume Examples: Skills
- Clinical Trial Management
- Regulatory Compliance
- Data Analysis
- Participant Recruitment
- Protocol Development
- Communication Skills
- Teamwork And Collaboration
- Problem-Solving Skills
- Time Management
- Attention To Detail
- Ethical Standards Adherence
- Patient Interaction
- Electronic Data Capture Systems
- Good Clinical Practice (GCP) Knowledge
- Project Management Skills
- Interpersonal Skills
Do notice that you should also include soft skills, as they’re also among the job requirements for clinical research coordinators.
Read more: Analytical Skills? Examples + Expert Advice
5. Add Other Sections to Your Clinical Research Coordinator Resume
To stand out as a candidate, consider enriching your resume with additional sections highlighting your breadth of experience and expertise. These extra elements can offer a fuller picture of who you are beyond your core job duties.
Consider these options to enhance your resume:
- List relevant certifications that add to your professional credibility, such as those in clinical research or data management.
- Include any relevant publications you've authored that pertain to clinical research, as they underscore your knowledge and authority in the field.
- If you've engaged in volunteer work related to clinical research, this can demonstrate your commitment and passion for the industry.
- Language skills can be an asset, especially in diverse work environments. Learn how to include language skills on your resume effectively.
Let’s go back to the topic of certifications for a moment, as they’re extremely important in your line of work.
One of the certificates you should consider in the first place is the Certified Clinical Research Coordinator (CCRC®) certification from ACRP which can elevate your resume and signal a high level of expertise. The ACRP credential verifies your proficiency in clinical trial coordination, regulatory compliance, and patient interaction, making it highly relevant in today’s clinical research landscape.
Read more: References on a Resume: The Ultimate Guide
6. Write a Clinical Research Coordinator Resume Summary or Resume Objective
Don't expect the hiring manager to peruse every detail of your resume. As revealed in our HR statistics report, they might only spend about 6 to 7 seconds on it. Capture their attention with a compelling resume introduction that highlights your key attributes.
This section is often referred to as a resume profile. Some liken it to an elevator pitch of yourself, but it's even more concise. Aim to capture their interest within a single paragraph.
If you possess over a year of experience, mention it. Include your job title, how you plan to contribute to the company, and a couple of notable achievements. This is known as a resume professional summary, and it should be prominently placed at the top.
For those pondering how to write a resume with no experience, the approach is similar but focuses on accomplishments from educational or personal projects. That’s why with little to no professional experience, you should write a clinical research coordinator resume objective.
Read more: Personal Mission Statement Tips
7. Write a Cover Letter for Your Clinical Coordinator Resume
In today's competitive job market, including a cover letter can set you apart from other applicants. A cover letter demonstrates your genuine interest in the position and the company, showing that you've taken the time to tailor your application specifically.
To craft a killer cover letter, follow these steps:
- Begin with a professional cover letter structure using the same header as your resume and a formal closing.
- Adhere to a proper cover letter format: keep it concise with 3–5 paragraphs, not exceeding one page.
- Start your cover letter by introducing your job title and adding an engaging opening line to captivate the reader.
- In the body, highlight your most significant achievements as a clinical research coordinator.
- Conclude with a persuasive cover letter closing statement that reaffirms your skills and suggests a meeting to discuss your potential contributions.
- Follow up with an application follow-up email weekly for up to a month, keeping it brief and attaching your resume and cover letter.
Read more: How to Write a Cover Letter: Everything You Should Know
Plus, a great cover letter that matches your resume will give you an advantage over other candidates. You can write it in our cover letter builder here. Here's what it may look like:
See more cover letter templates and start writing.
Remember, your clinical research coordinator's resume is your professional introduction. Keep refining it until it truly represents your skills and achievements. Thank you for reading, and feel free to leave any questions regarding your clinical research coordinator resume in the comments.
About Zety’s Editorial Process
This article has been reviewed by our editorial team to make sure it follows Zety's editorial guidelines. We’re committed to sharing our expertise and giving you trustworthy career advice tailored to your needs. High-quality content is what brings over 40 million readers to our site every year. But we don't stop there. Our team conducts original research to understand the job market better, and we pride ourselves on being quoted by top universities and prime media outlets from around the world.